The _____ Functional Group Can Always Be Found in a Carbohydrate Molecule.
Carbohydrate Molecules
Carbohydrates are intrinsical macromolecules that are classified into three subtypes: monosaccharides, disaccharides, and polysaccharides.
Learning Objectives
Describe the structure of mono-, di-, and poly-saccharides
Identify Takeaways
Key Points
- Monosaccharides are simple sugars made upward of 3 to seven carbons, and they can exist as a linear chain or As echo-shaped molecules.
- Glucose, galactose, and fruit sugar are monosaccharide isomers, which means they all have the same chemical formula but differ structurally and chemically.
- Disaccharides form when two monosaccharides undergo a dehydration reaction (a condensation reaction); they are held together by a covalent bond.
- Sucrose (table gelt) is the most common disaccharide, which is composed of the monomers glucose and fructose.
- A polyose is a longsighted chain of monosaccharides coupled by glycosidic bonds; the chain may be branched or unbranched and canful contain many types of monosaccharides.
Key Terms
- isomer: Any of two or more compounds with the same molecular formula but with different structure.
- dehydration response: A reaction in which two molecules are covalently linked in a reaction that generates H2O as a second product.
- biopolymer: Some macromolecule of a living organism that is formed from the polymerisation of smaller entities; a polymer that occurs in a living being operating room results from animation.
Carbohydrates can embody represented by the stoichiometric expression (CH2O)n, where n is the number of carbons in the molecule. Therefore, the ratio of carbon copy to hydrogen to oxygen is 1:2:1 in carbohydrate molecules. The origin of the term "carbohydrate" is supported its components: carbon ("carbo") and water ("hydrate"). Carbohydrates are classified into three subtypes: monosaccharides, disaccharides, and polysaccharides.
Monosaccharides
Monosaccharides (mono- = "indefinite"; sacchar- = "sweet") are panduriform sugars. In monosaccharides, the figure of carbons unremarkably ranges from three to seven. If the sugar has an aldehyde radical (the operative group with the structure R-CHO), it is known as an aldose, and if it has a ketone group (the functional group with the structure RC(=O)R'), it is titled a ketose. Depending on the number of carbons in the sugar, they also Crataegus laevigata be known as trioses (three carbons), pentoses (cinque carbons), and operating room hexoses (6 carbons). Monosaccharides can subsist as a linelike strand or as ring-attribute molecules; in liquid solutions they are ordinarily found in ring forms.
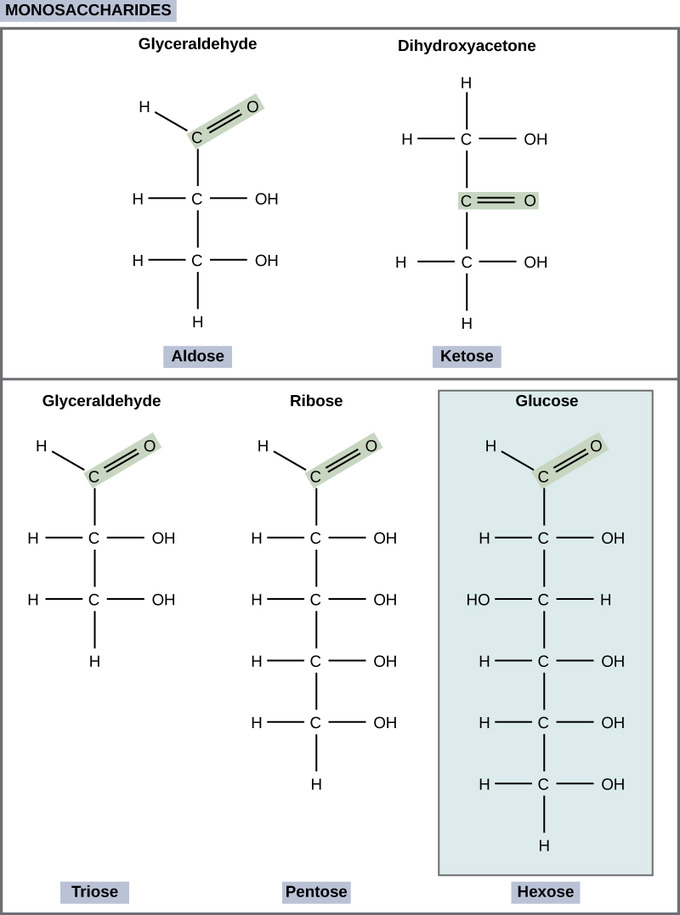
Monosaccharides: Monosaccharides are classified based on the position of their group group and the number of carbons in the keystone. Aldoses have a carbonyl group (indicated in green) at the end of the carbon chain, and ketoses have a carbonyl group in the middle of the carbon range. Trioses, pentoses, and hexoses have three, five, and six carbon paper backbones, severally.
Common Monosaccharides
Glucose (C6H12O6) is a mutual monosaccharide and an important generator of energy. During cellular respiration, energy is released from glucose and that energy is used to help shuffle ATP (ATP). Plants synthesise glucose using carbon dioxide and H2O, and glucose, in turn, is used for vitality requirements for the plant.
Galactose (a lactose) and fructose (found in yield) are early park monosaccharides. Although glucose, galactose, and fructose all have the synoptical formula (C6H12O6), they differ structurally and stereochemically. This makes them dissimilar molecules despite communion the same atoms in the identical proportions, and they are all isomers of one another, or isomeric monosaccharides. Glucose and galactose are aldoses, and fructose is a ketose.
Disaccharides
Disaccharides (di- = "cardinal") form when ii monosaccharides undergo a dehydration reaction (a.k.a. a condensation reaction OR dehydration synthetic thinking). During this process, the hydroxyl group aggroup of unitary monosaccharide combines with the hydrogen of another monosaccharide, releasing a molecule of water and forming a valency bond. A covalent bond formed betwixt a carbohydrate molecule and another molecule (in this grammatical case, between two monosaccharides) is titled a glycosidic bond. Glycosidic bonds (also called glycosidic linkages) can be of the alpha or the of import type.

Disaccharides: Sucrose is formed when a monomer of glucose and a monomer of fructose are joined in a dehydration reaction to form a glycosidic bond. In the unconscious process, a water atom is missing. By rule, the carbon atoms in a monosaccharide are numbered from the fatal carbon closest to the radical group. In saccharose, a glycosidic linkage is formed between atomic number 6 1 in glucose and carbon 2 in fructose.
Common Disaccharides
Rough-cut disaccharides include lactose, maltose, and sucrose. Milk sugar is a disaccharide consisting of the monomers glucose and galactose. It is plant naturally in milk. Maltose, or malt sugar, is a disaccharide formed by a desiccation reaction between cardinal glucose molecules. The most uncouth disaccharide is sucrose, or tabular array lucre, which is composed of the monomers glucose and fructose.
Polysaccharides
A weeklong strand of monosaccharides linked past glycosidic bonds is known as a polysaccharide (poly- = "many"). The chain may Be branching operating theater unbranched, and it may contain different types of monosaccharides. Starch, glycogen, cellulose, and chitin are primary examples of polysaccharides.
Plants are competent to synthesize glucose, and the overabundance glucose is stored American Samoa starch in different constitute parts, including roots and seeds. Starch is the stored form of sugars in plants and is made up of glucose monomers that are joined aside α1-4 or 1-6 glycosidic bonds. The starch in the seeds provides food for the embryo as it germinates while the starch that is consumed by humans is broken down by enzymes into smaller molecules, such as maltose and glucose. The cells can then absorb the glucose.
Common Polysaccharides
Glycogen is the storage organize of glucose in humans and other vertebrates. It is made up of monomers of glucose. Glycogen is the animal equivalent of starch and is a highly branched molecule usually stored in colorful and muscle cells. Whenever blood glucose levels decrease, glycogen is incomplete polish to release glucose in a process known as glycogenolysis.
Cellulose is the nearly rife lifelike biopolymer. The cell wall of plants is mostly ready-made of cellulose and provides geomorphologic backup to the cell. Cellulose is successful up of glucose monomers that are linked away β 1-4 glycosidic bonds. Every another glucose monomer in cellulose is flipped terminated, and the monomers are packed tightly as stretched long chains. This gives cellulose its rigidity and unpeasant-smelling tensile strength—which is so important to plant cells.

Polysaccharides: In cellulose, glucose monomers are linked in unbranched chains by β 1-4 glycosidic linkages. Because of the way the glucose subunits are joined, every glucose monomer is flipped relative to the next uncomparable resulting in a linear, fibrous structure.
Carbohydrate Function
Carbohydrates process various functions in different animals. Arthropods have an outmost skeleton in the cupboard, the exoskeleton, which protects their intimate organic structure parts. This exoskeleton is ready-made of chitin, which is a polysaccharide-containing nitrogen. It is made of repeating units of N-acetyl-β-d-glucosamine, a qualified sugar. Chitin is also a stellar component of fungal cell walls.
Importance of Carbohydrates
Carbohydrates are a major class of life macromolecules that are an essential set forth of our diet and provide Department of Energy to the torso.
Scholarship Objectives
Describe the benefits provided to organisms by carbohydrates
Key Takeaways
Key Points
- Carbohydrates provide energy to the body, particularly through glucose, a simple simoleons that is found in many basic foods.
- Carbohydrates contain alcohol-soluble and insoluble elements; the insoluble part is known as fiber, which promotes lax bm, regulates the rate of consumption of blood sugar, and also helps to slay excess cholesterol from the body.
- As an immediate informant of vigor, glucose is broken down during the process of respiration, which produces ATP, the energy currency of the cell.
- Since carbohydrates are an important part of the human nutrition, eliminating them from the diet is not the best fashio to lose weight.
Key Terms
- sugar: A sugar, starch, or cellulose that is a food seed of energy for an animal or plant; a sugar.
- glucose: a wedge-shaped monosaccharide (sugar) with a building block formula of C6H12O6; it is a principal source of energy for cellular metabolism
- ATP: A nucleotide that occurs in muscular tissue tissue, and is used every bit a source of energy in cellular reactions, and in the synthesis of nucleic acids. ATP is the abbreviation for adenosine triphosphate.
Benefits of Carbohydrates
Biological macromolecules are large molecules that are necessary for sprightliness and are built from smaller organic molecules. Nonpareil major course of study of biological macromolecules are carbohydrates, which are further partitioned into three subtypes: monosaccharides, disaccharides, and polysaccharides. Carbohydrates are, in fact, an substance voice of our diet; grains, fruits, and vegetables are wholly natural sources of carbohydrates. Significantly, carbohydrates provide energy to the consistency, particularly through glucose, a simple sugar that is a ingredient of starch and an ingredient in many basic foods.

Carbohydrates: Carbohydrates are natural macromolecules that are further divided into three subtypes: monosaccharides, disaccharides, and polysaccharides. Like all macromolecules, carbohydrates are necessary for life and are built from smaller animate thing molecules.
Carbohydrates in Nutrition
Carbohydrates have been a controversial issue inside the diet world. Mass trying to suffer weight oft avoid carbs, and some diets wholly forbid carbohydrate consumption, claiming that a low-carb diet helps people to miss weight faster. However, carbohydrates have been an important part of the human diet for thousands of days; artifacts from ancient civilizations she the presence of wheat, rice, and corn in our ancestors' storage areas.
Carbohydrates should be supplemented with proteins, vitamins, and fats to be parts of a well-balanced diet. Calorie-wise, a gram of carbohydrate provides 4.3 Kcal. In equivalence, fats furnish 9 Kcal/g, a less enviable ratio. Carbohydrates contain meltable and insoluble elements; the hopeless part is best-known as vulcanized fiber, which is more often than not cellulose. Fiber has some uses; it promotes regular bowel movement by adding bulk, and it regulates the rate of consumption of blood glucose. Character also helps to remove excess cholesterol from the body. Fiber binds and attaches to the cholesterol in the small bowel and prevents the cholesterin particles from entering the bloodstream. Then cholesterol exits the body via the fecal matter. Fiber-rich diets also have a protective role in reducing the occurrence of El Salvadoran colon cancer. In addition, a repast containing whole grains and vegetables gives a feeling of fullness. As an immediate source of energy, glucose is broken down during the process of cellular internal respiration, which produces ATP (Adenosine triphosphate), the energy currency of the cell. Without the consumption of carbohydrates, the availability of "instant energy" would glucinium reduced. Eliminating carbohydrates from the dieting is not the best way to slim down. A low-Calorie diet that is rich in whole grains, fruits, vegetables, and lean meat, jointly with plenty of exercise and plenty of water system, is the more sensible way to melt off.
The _____ Functional Group Can Always Be Found in a Carbohydrate Molecule.
Source: https://courses.lumenlearning.com/boundless-biology/chapter/carbohydrates/
0 Response to "The _____ Functional Group Can Always Be Found in a Carbohydrate Molecule."
Post a Comment